UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_____________________
FORM 8-K
_____________________
CURRENT REPORT
Pursuant to Section 13 OR 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): June 20, 2019
_____________________
AXCELLA HEALTH INC.
(Exact name of registrant as specified in its charter)
________________________
Delaware | 001-38901 | 26-3321056 | ||
(State or other jurisdiction of incorporation) | (Commission File Number) | (IRS Employer Identification No.) | ||
840 Memorial Drive Cambridge, Massachusetts | 02139 |
(Address of principal executive offices) | (Zip Code) |
Registrant's telephone number, including area code: (857) 320-2200
Not Applicable
(Former name or former address, if changed since last report)
________________________
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions ( see General Instruction A.2. below):
☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company x
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
Common Stock, $0.001 Par Value | AXLA | Nasdaq Global Market |
Item 2.02. Results of Operations and Financial Condition.
On June 20, 2019, Axcella Health Inc. announced its financial results for the first quarter ended March 31, 2019. The full text of the press release issued in connection with the announcement is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information in this Form 8-K (including Exhibit 99.1) shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits:
Exhibit No. | Description | |
99.1 | Press Release issued by Axcella Health Inc., dated June 20, 2019, furnished herewith | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
AXCELLA HEALTH INC. | ||||||
Date: June 20, 2019 | By: | /s/ William R. Hinshaw, Jr. | ||||
William R. Hinshaw, Jr. | ||||||
Chief Executive Officer, President and Director | ||||||
Exhibit 99.1
Axcella Health Reports First Quarter 2019 Financial Results
and Provides Company Update
• | Successfully completed initial public offering, raising $71.4 million in gross proceeds |
• | Presented data on AXA1665 and AXA1125 at two leading medical conferences |
• | Identified drug development path for AXA1665 following the FDA meeting in March |
• | Initiated two Non-IND, IRB-Approved Clinical Studies for AXA1665, AXA1125 and AXA1957 |
• | Expanded leadership team and announced two patent issuances |
Cambridge, Mass., June 20, 2019 – Axcella Health Inc. (Nasdaq: AXLA) (“Axcella” or the “Company”), a biotechnology company pioneering the research and development of novel multifactorial interventions to address dysregulated metabolism and support health, today announced financial results for the first quarter ended March 31, 2019 and provided a company update.
“We are excited by the progress our organization has made across multiple fronts during the first quarter of 2019. We presented new data from both our AXA1665 program for Hepatic Encephalopathy and AXA1125 program in liver that we believe demonstrate the strength of our AXA development platform and potential of endogenous metabolic modulators. We also continued to strengthen our patent portfolio and made key senior level hires. All of these accomplishments successfully positioned Axcella to complete an initial public offering with gross proceeds of $71.4 million in May of this year,” said Bill Hinshaw, President and Chief Executive Officer of Axcella. “We look forward to using these proceeds to continue the rapid development of our first four AXA candidates, in addition to potentially expanding into new areas, including liver and blood, as we strive to become the preeminent biotechnology company reprogramming metabolism to address a diverse set of complex diseases and support health.”
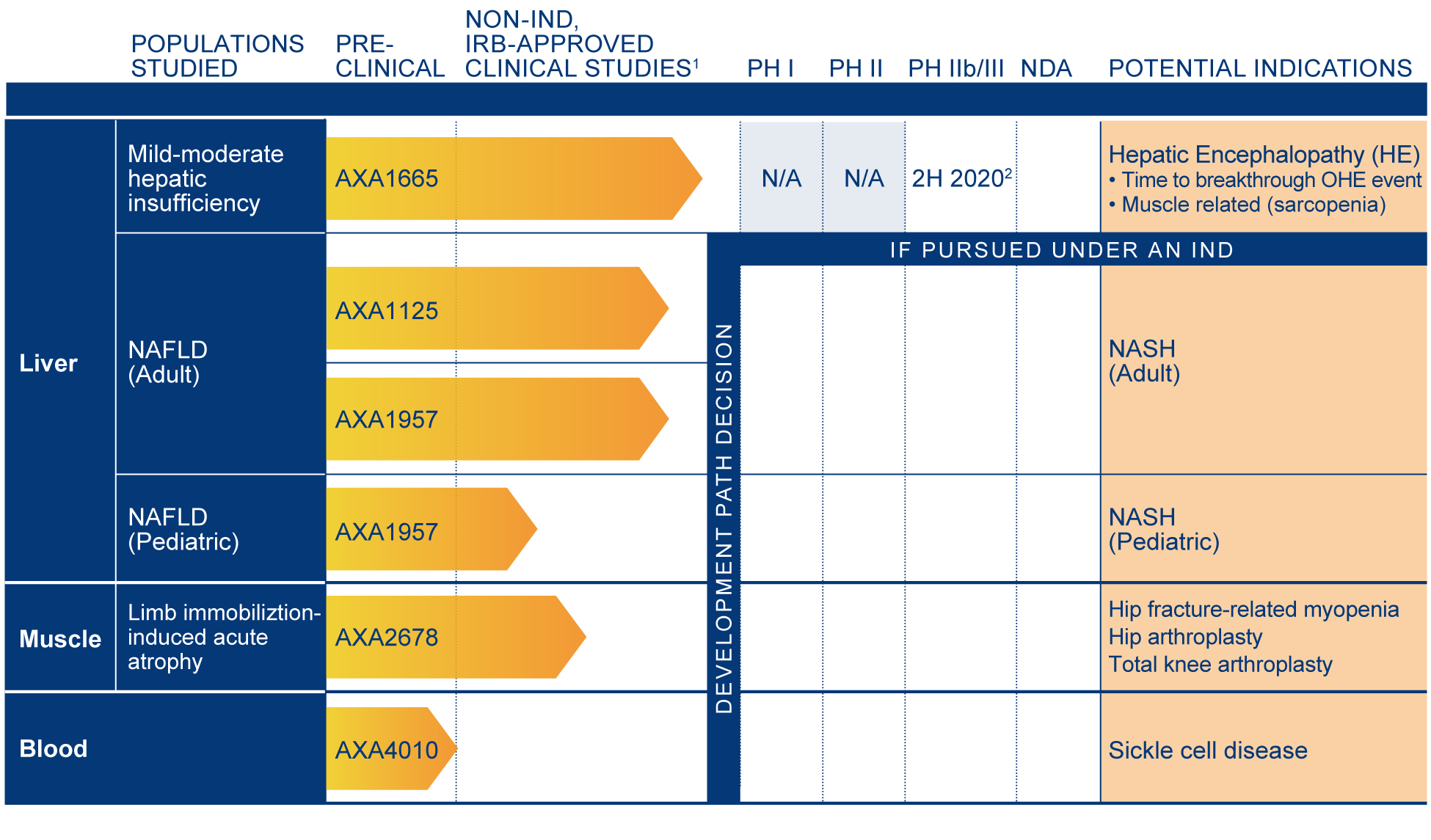
(1) In the above pipeline chart, “Development Path Decision” reflects the point in a program at which we decide whether to develop an AXA Candidate as a drug product candidate under an Investigational New Drug Application (IND), develop it as a non‑drug product candidate or terminate development. We have made a decision to develop AXA1665 as a drug product candidate and anticipate interacting with the U.S. Food and Drug Administration (FDA) again prior to submitting an IND. We have not made a development path decision for any of our other AXA Candidates.
(2) We believe that this human clinical study under an IND (Clinical Trial) has the potential to serve as a registrational (pivotal) Clinical Trial, subject to continuing IND discussions and allowance by the FDA.
Definitions: IRB = Institutional Review Board; NAFLD = non‑alcoholic fatty liver disease; OHE = overt hepatic encephalopathy; NASH = nonalcoholic steatohepatitis.
(2) We believe that this human clinical study under an IND (Clinical Trial) has the potential to serve as a registrational (pivotal) Clinical Trial, subject to continuing IND discussions and allowance by the FDA.
Definitions: IRB = Institutional Review Board; NAFLD = non‑alcoholic fatty liver disease; OHE = overt hepatic encephalopathy; NASH = nonalcoholic steatohepatitis.
Clinical Highlights
• | In April 2019, Axcella presented late-breaker data at The International Liver Congress™ 2019 (EASL) demonstrating that AXA1665, Axcella’s lead AXA™ candidate, positively impacted markers related to liver health in subjects with mild and moderate hepatic insufficiency, including: |
o | Increased basal (fasted) Fischer’s ratio, the molar ratio of branched chain amino acids to aromatic amino acids, which appears to have prognostic significance in subjects with cirrhosis and end-stage liver disease |
o | Decreased plasma ammonia area under the curve, suggesting that blood ammonia levels did not increase despite the administration of an added nitrogen load |
o | Tended to maintain a leaner (dry lean mass, lean body mass, and skeletal muscle mass) phenotype with the average Liver Frailty Index (LFI) score directionally moving toward less frailty compared to baseline |
• | In January 2019, Axcella presented mechanistic data demonstrating that AXA1125 simultaneously modulated hepatic metabolic, inflammatory and fibrotic biochemical nodes in primary human single- and multi-cellular systems at the Integrated Pathways of Disease in NASH and NAFLD Keystone Symposium and was included among the highlighted presentations. AXA1125 data presented included: |
o | Lowered triglycerides in hepatocytes (metabolic node) |
o | Suppressed aerobic glycolysis while preserving total ATP levels; and reduced proinflammatory cytokines in macrophages (inflammation node) |
o | Reduced ProC3 protein secretion and other key fibrogenic markers, including reducing the activation and proliferation of stellate cells (fibrosis node) |
o | Multimodal effects were seen (or manifested) with AXA1125 consistently across cells, animal models, humans |
• | Following a meeting with the FDA in March, Axcella decided to pursue a drug development path for AXA1665. Under the planned IND, the initial indication would be for the treatment of HE |
o | A Phase IIb/III Clinical Trial is anticipated to launch in the second half of 2020 and could potentially serve as a registrational (pivotal) trial to support the submission of a New Drug Application, or NDA |
• | During the first quarter of 2019, Axcella initiated two Non-IND, IRB-Approved Clinical Studies |
o | Non-IND, IRB-Approved Clinical Study of AXA1665 |
o | Non-IND, IRB-Approved Clinical Study of AXA1125 and AXA1957 |
Corporate Highlights
• | In May 2019, Axcella completed its initial public offering, raising gross proceeds of $71.4 million through the sale of 3,571,428 shares of common stock at an initial public offering price of $20.00 per share and commenced trading on The Nasdaq Global Market under the ticker symbol AXLA |
• | In March 2019, Axcella expanded its leadership team with the appointments of Shreeram Aradhye, M.B.B.S., M.D., as Executive Vice President, Chief Development Officer and Karen Lewis as Senior Vice President, Human Resources |
• | In February and April 2019, Axcella announced two patent issuances for AXA1125 covering composition of matter and methods of use in liver dysfunction and liver disease |
Anticipated Milestones
• | Initiate a Non-IND, IRB-Approved Clinical Study of AXA1957 in adolescent subjects with NAFLD in the second half of 2019 |
• | Initiate a Non-IND, IRB-Approved Clinical Study of AXA4010 in subjects with sickle cell disease in the second half of 2019 |
• | Report data from our Non-IND, IRB-Approved Clinical Study of AXA1665 in subjects with hepatic insufficiency in the first half of 2020 |
• | Report data from our Non-IND, IRB-Approved Clinical Study of AXA1125 and AXA1957 in adult subjects with NAFLD in the second half of 2020 |
• | Report data from our Non-IND, IRB-Approved Clinical Study of AXA1957 in adolescent subjects with NAFLD in the second half of 2020 |
• | Report data from our Non-IND, IRB-Approved Clinical Study of AXA4010 in subjects with sickle cell disease in the second half of 2020 |
First Quarter 2019 Financial Results
For the first quarter ended March 31, 2019, Axcella reported a net loss of approximately $11.6 million, or $2.43 per share, basic and diluted, compared to a net loss for the quarter ended March 31, 2018 of $8.1 million, or $1.92 per share, basic and diluted.
Total operating expenses for the quarter ended March 31, 2019 were $11.0 million compared to $7.6 million for the quarter ended March 31, 2018. Included in total operating expense for the first quarter of 2019 were $1.1 million of non-cash stock-based compensation expenses, compared to $0.4 million of non-cash stock-based compensation in the prior year period.
Research and development expenses for the quarter ended March 31, 2019 were $7.6 million, compared to $5.5 million for the quarter ended March 31, 2018. The increase in expense for the quarter was driven by an increase in costs related to the conduct of Non-IND, IRB-Approved Clinical Studies and other expenses associated with the development of AXA candidates in 2019.
General and administrative expenses were $3.5 million for the quarter ended March 31, 2019, compared to $2.1 million for the quarter ended March 31, 2018. The increase in general and administrative expenses for the year was driven by increased professional services and employee-related costs as the Company continues to increase headcount and expand infrastructure to support its growth.
Cash and cash equivalents were $66.8 million as of March 31, 2019.
About Axcella Health
Axcella is designing and developing AXA Candidates, compositions of endogenous metabolic modulators, or EMMs, engineered in distinct ratios, designed to target and maximize the fundamental role that EMMs play in regulating multiple metabolic functions. Axcella’s AXA Candidates are generated from its proprietary, human-focused AXA Development Platform. Axcella believes its expertise and capabilities in EMMs position it to become a preeminent biotechnology company reprogramming metabolism to address a diverse set of complex diseases and support health. Axcella’s AXA Development Platform has already produced a pipeline of product candidates in programs targeting liver, muscle and blood. Axcella was founded by Flagship Pioneering.
About Non-IND, IRB-Approved Clinical Studies
Axcella conducts non-investigational new drug application (Non-IND), Institutional Review Board (IRB)-approved clinical studies in humans with its AXA Candidates under U.S. Food and Drug Administration regulations and guidance supporting research with food outside of an IND. In these studies, Axcella evaluates in humans, including in individuals with disease, AXA Candidates for safety, tolerability and effects on the normal structures and functions of the body. Non-IND, IRB-Approved Clinical Studies are not designed or intended to evaluate an AXA Candidate’s ability to diagnose, cure, mitigate, treat or prevent a disease. If Axcella decides to further develop an AXA Candidate as a potential therapeutic, subsequent studies will be conducted under an IND.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, statements regarding the development potential of our current AXA candidates, potential expansion into new therapeutic fields, the timing of our clinical studies and the timing of receipt of data from the same, our liquidity and our strategy, business plans and focus. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Any forward-looking statements in this press release are based on management’s current expectations and beliefs and are subject to a number of risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained in this press release, including, without limitation, those related to the breadth of our pipeline of product candidates, the strength of our proprietary product platform, the efficiency of our discovery and development approach, the clinical development and safety profile of our AXA Candidates and their therapeutic potential, whether and when, if at all, our AXA Candidates will receive approval from the U.S. Food and Drug Administration and for which, if any, indications, competition from other biotechnology companies, our liquidity, our ability to successfully develop our AXA Candidates through current and future milestones on the anticipated timeline, if at all, past results from Non-IND, IRB-Approved Clinical Studies not being representative of future results, and other risks identified in our SEC filings, including our final prospectus for our initial public offering, and subsequent filings with the SEC. We caution you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. We disclaim any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. Any forward-looking statements contained in this press release represent our views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date. We explicitly disclaim any obligation to update any forward-looking statements.
Axcella Health Inc. | |||||||||||||
Condensed Consolidated Statements of Operations (Unaudited) | |||||||||||||
(in thousands, except share and per share data) | |||||||||||||
Three Months Ended March 31, | |||||||||||||
2019 | 2018 | ||||||||||||
Operating expenses: | |||||||||||||
Research and development | $ | 7,563 | $ | 5,455 | |||||||||
General and administrative | 3,468 | 2,136 | |||||||||||
Total operating expenses | 11,031 | 7,591 | |||||||||||
Loss from Operations | (11,031) | (7,591) | |||||||||||
Other (expense) income | (542) | (509) | |||||||||||
Net loss | $ | (11,573 | ) | $ | (8,100 | ) | |||||||
Net loss per share, basic and diluted | $ | (2.43 | ) | $ | (1.92 | ) | |||||||
Weighted average common shares outstanding, basic and diluted | 4,775,828 | 4,229,118 | |||||||||||
Axcella Health Inc. | |||||||||||
Condensed Consolidated Balance Sheet Data (Unaudited) | |||||||||||
(in thousands) | |||||||||||
March 31, | December 31, | ||||||||||
2019 | 2018 | ||||||||||
Assets: | |||||||||||
Cash and cash equivalents | $ | 66,769 | $ | 79,466 | |||||||
Other assets | 4,149 | 2,378 | |||||||||
Total assets | $ | 70,918 | $ | 81,844 | |||||||
Liabilities and stockholders' (deficit) equity | |||||||||||
Liabilities | $ | 33,253 | $ | 33,755 | |||||||
Preferred stock | 197,888 | 197,842 | |||||||||
Stockholders' (deficit) equity | (160,223 | ) | (149,753 | ) | |||||||
Total liabilities and stockholders' equity | $ | 70,918 | $ | 81,844 | |||||||
Investor Contact | Company Contact | |
Alison Williams | Lauren Stival | |
awilliams@axcellahealth.com | ir@axcellahealth.com | |
(857) 320-2204 | 212-698-8646 | |
